Carbohydrates as well as proteins are polymers and contain only a few different types of atom. In the case of carbohydrates, the basic molecular units are called monosaccharides – these are the monomers. (mono = single; poly = multiple; saccharide = sugar)
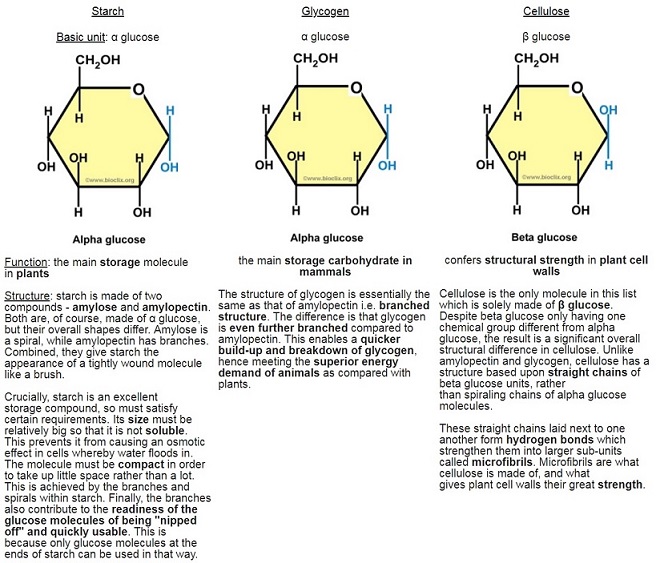
α (alpha) glucose is the most important monosaccharide to learn, as you need to be able to draw it:
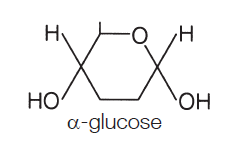
The points where the lines intersect each symbolise a carbon (C) atom. You need not show those. The figure above is taken from the specification itself, so take it as a good guide. So the monosaccharide alpha glucose (commonly, just glucose) somehow becomes a polysaccharide, This is achieved by condensation reactions, and the bonds formed are called glycosidic bonds.
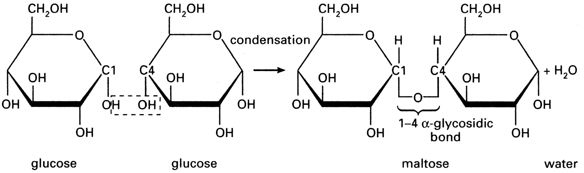
You should be able to draw this. The resulting molecule, maltose, is a disaccharide (two monomers). If you keep adding glucose molecules to the chain, you get… *drum roll please* …starch. Starch is made up of multiple (very many indeed) monomers, so it is a polymer i.e. it is made of multiple monosaccharides, so it is a polysaccharide.
Potatoes anyone?
You also need to know about two other disaccharides and their constituent monosaccharides – sucrose and lactose
Sucrose is made of glucose and fructose.
Lactose is made of glucose and galactose.
It’s easy enough to remember: they’re both made of glucose, and lactose (MILK) is also made of galactose (galaxy – Milky Way). Both sucrose and lactose are formed similarly by the condensation of their monosaccharides.
During digestion, these large molecules must be broken back down into small molecules. Starch must be broken down to glucose. Enzymes produced by the salivary glands and the pancreas carry out this reaction in the mouth and stomach. One particular such enzyme is amylase. By the time food reaches the small intestine where nutrients are absorbed, glucose on its own must be made available for absorption in the epithelial cells lining the intestine. This is achieved by maltase, which breaks down… that’s right, maltose, leaving behind single molecules of glucose.
Regarding lactose you need to be aware of lactose intolerance. Just as for starch or maltose, lactose too must be broken down successfully for its monomers (glucose and galactose) to be absorbed by the body. Not surprisingly, this is achieved by the enzyme lactase. People deficient of this enzyme will not properly digest, or break down lactose from milk, so lactose will go unchecked into the large intestine, where microorganisms will use it for respiration, leaving behind a lot of gas. This causes symptoms like bloating, abdominal pain and diarrhoea.
Sugars can be reducing or non-reducing, depending on their chemistry (you do not want to go into that. Unless you’re into chemistry). Reducing sugars are ALL monosaccharides, and the disaccharides lactose and maltose. All else including sucrose is non-reducing. Benedict’s reagent is a test for reducing and non-reducing sugars.
Benedict’s reagent = light blue
You throw some glucose into the mix = orange/brick red due to it being reducing (reduces copper ions Cu2+ to Cu+)
You become adventurous and throw some sucrose into the mix = ends it utter disappointment when you get nothing, light blue, due to it not being reducing.
And finally, there is a test for starch. Chop a potato (they are practically made of the stuff), add iodine which is yellow. The starch in the potato will turn it blue. This is a very simple test for starch – if the solution stays yellow it’s negative, if it goes blue, it’s positive.