AQA A-Level Biology
-
Sample content (FREE TO VIEW)5 Lessons
-
Biological Molecules10 Lessons
-
Cells6 Lessons
-
Organisms exchange substances with their environment5 Lessons
-
Genetic information, variation and relationships between organisms7 Lessons
-
Energy transfers in and between organisms4 Lessons
-
Organisms respond to changes in their internal and external environments9 Lessons
-
? Survival and response
-
? Receptors
-
? Control of Heart Rate
-
⚡ Nerve Impulses
-
? Synaptic Transmission
-
?♂️ Skeletal muscles are stimulated to contract by nerves and act as effectors
-
✋ Principles of homeostasis and negative feedback
-
?️ Control of Blood Glucose Concentration
-
⚖️ Control of blood water potential
-
? Survival and response
-
Genetics, populations, evolution and ecosystems4 Lessons
-
The control of gene expression8 Lessons
-
? Alteration of the sequence of bases in DNA can alter the structure of proteins
-
? Most of a Cell's DNA is not Translated
-
? Regulation of Transcription and Translation
-
?⚕️ Gene expression and cancer
-
? Using genome projects
-
? Recombinant DNA Technology
-
? Differences in DNA between individuals of the same species can be exploited for identification and diagnosis of heritable conditions
-
?️♂️ Genetic Fingerprinting
-
? Alteration of the sequence of bases in DNA can alter the structure of proteins
? Proteins (FREE SAMPLE)
The A Level Biologist - Your Hub February 12, 2020
Proteins are at the heart of living organisms. Their functions are very varied, from the hair on your head, to the haemoglobin in your red blood cells (which carries oxygen around the body), to the claws of a lion, to insulin (blood glucose regulation). All these highly varied proteins are made of their building blocks – amino acids . This is what the generalised structure of an amino acid looks like (make sure you can draw this):
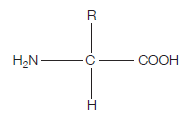
If you’re wondering what this actually is, read on. The clues are in the name (as they usually are).
AMINO – the H2N on the left hand side is an amino group ACID – the COOH on the right hand side is a carboxylic acid group (simply an acid).
The hydrogen (H) on the bottom is there all the time (just like the amino group and the acid group), while the R group is the variable which determines what particular amino acid this will be. For example, if the R group was a hydrogen, the amino acid would be glycine .
The next diagram shows condensation, and the subsequent formation of a bond between two amino acids (any two). This bond is a peptide bond. The resulting molecule is called a polypeptide.
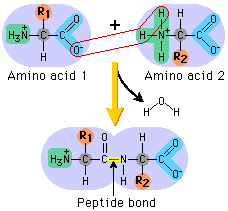
This video is an excellent tool for understanding the processes by which these amino acids end up in highly structured, complex proteins with varied and important functions within organisms:
Proteins have a primary, secondary, tertiary and (some only) quaternary structure. The tertiary structure of proteins is their 3D shape which is highly folded and has a unique structure. This structure gives proteins their specific function. For example, if insulin was misfolded, it would cease to function properly. Of course though, the origin of misfolding is likely to be in the primary structure, due to a mutation.
For example, if the gene responsible for coding the amino acid sequence for insulin was mutated, then the insulin’s primary structure (which is the string of amino acids) would be different, leading to a different secondary structure, tertiary structure, and ultimately, a lack of proper function.
NB: The tertiary structure of proteins determines their proper function .
You also need to know about the Biuret test for proteins. Biuret is a pale blue solution which, when added to protein, will turn lilac .
The sample to be tested could be taken from a certain food for example. Have a look:

