Urinogenital systems
Urinogenital (a.k.a. urogenital; genitourinary) systems play roles in excretion (releasing urine produced by the kidneys) and reproduction via the genitals. The grouping arises due to the physical proximity of the systems, their common developmental pathway, and some of their shared pathways.
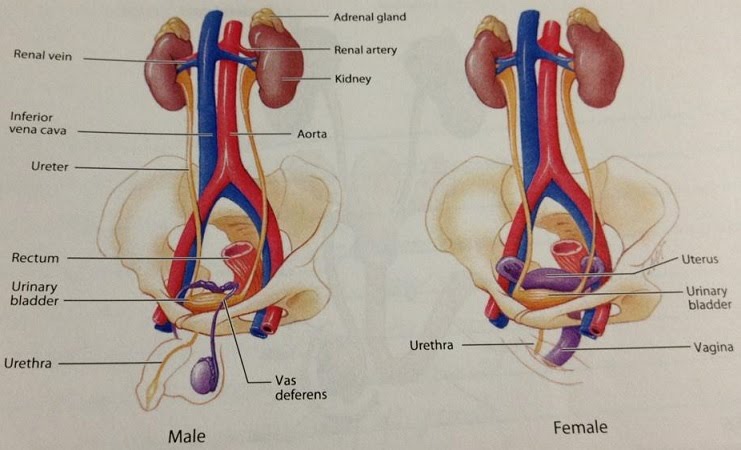
The male urethra is a shared pathway for urine during excretion as well as semen during ejaculation. The kidneys are connected to the bladder via the ureters. The urethra develops further out of the bladder and provides the exit path for urine. In the female urinogenital system the urethra is separate, lying between the clitoris and the vagina.
Human gonads
The gonads – ovaries and testes – produce gametes, the haploid sexual reproduction cells made through meiosis, which upon fertilisation complete the chromosome set and start the development of a new human. The gonads are part of the reproductive system which contains other organs with varying functions to assist sexual reproduction.
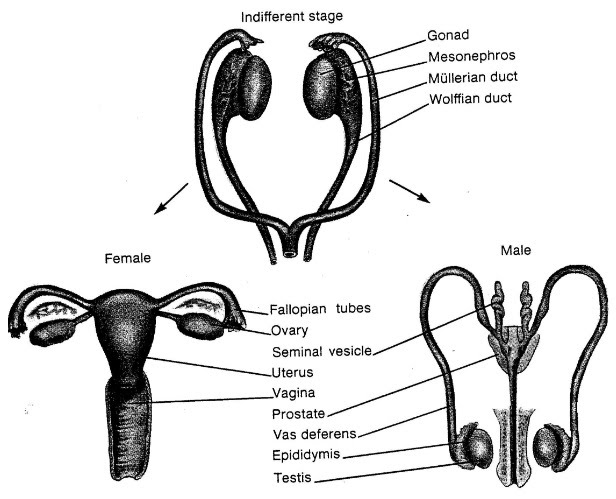
Gonads differentiate before birth in humans, into testes or ovaries.
Ovaries produce egg cells released one (sometimes two! or many more if induced by hormones e.g. IVF) at a time, which travel from each ovary (they take turns to release an egg) down the Fallopian tube towards the uterus. The egg can undergo fertilisation by a sperm cell found in the Fallopian tube, and develop into a zygote, attach to the upper lining of the womb (endometrium) and start pregnancy; or be shed alongside part of the endometrium two weeks after ovulation (egg release from the ovary) – this is menstruation.
Testes produce millions and billions of precursor sperm cells, only half of which mature into viable sperm cells in a process spanning 2-3 months. The cells are stored in the epididymis before travelling through the vas deferens towards the prostate and seminal vesicle where the cells mix with alkaline seminal fluid to form the solution that gets expelled by smooth muscles through the penis during ejaculation. Hence, the sperm cells leave the body. Their conception route to the egg in the Fallopian tube can be via the vagina during intercourse.
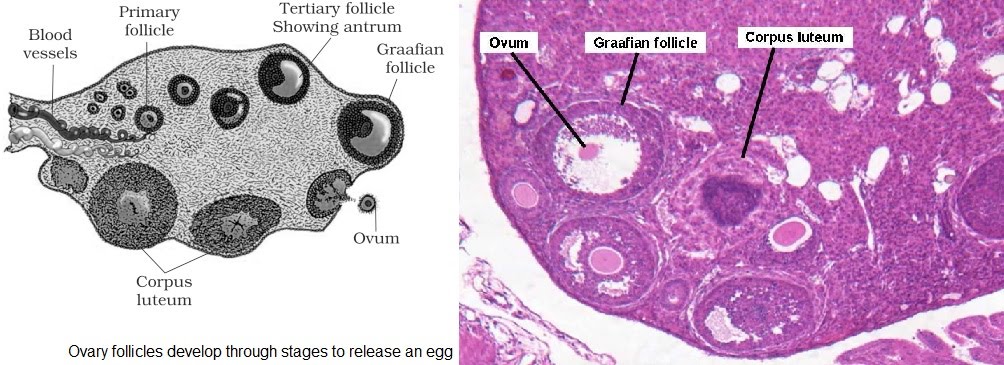
Eggs (ova) are released following follicle maturation in the ovary. The follicle develops, acquires fluid (antrum) and releases an ovum into the Fallopian tube, prior to the involution of the leftover tissue i.e.corpus luteum.
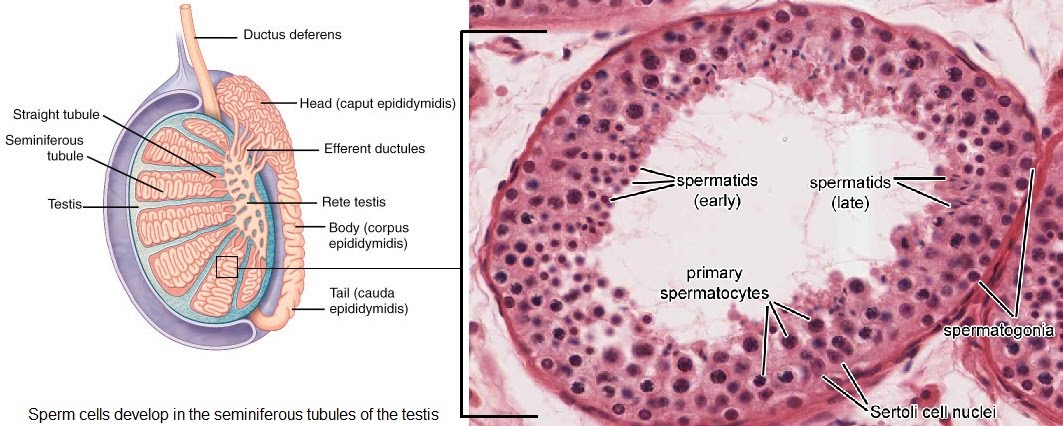
Sperm development takes place in the seminiferous tubules of the testis. From the outside in, the different stages of development are followed through.
Gamete development
Meiosis enables the production of cells with half the genetic material of the parent cell. This in turn enables the joining together of two haploid cells to form a zygote that has the full number of chromosomes again e.g. diploid in humans.
Gametes are these cells that are haploid are are used as part of sexual reproduction in mammals to enable more genetically diverse offspring, compared with asexual reproduction. Gametes are egg and sperm cells. Their development through meiosis involves multiple stages, dispersed throughout an individual’s lifetime as we will see for eggs which finish the first part of development even prior to the birth of the individual who may use them later.
The precursor (primordial) cells of eggs are oogonia (oogonium, sg.) and those of sperm are spermatogonia (spermatogonium, sg.). These grow and divide (mitosis) before undergoing meiosis I, maturing into oocytes and spermatocytes. These cells are termed primary, while the resulting cells after meiosis I are termed secondary.
So primary spermatocyes undergo the first stage of meiosis, producing secondary spermatocytes. Primary oocytes divide unevenly, producing one large offspring cell which is the secondary oocyte and a smaller cell which doesn’t go any further in the process, called a first polar body (yes, there will be a second polar body during meiosis II).
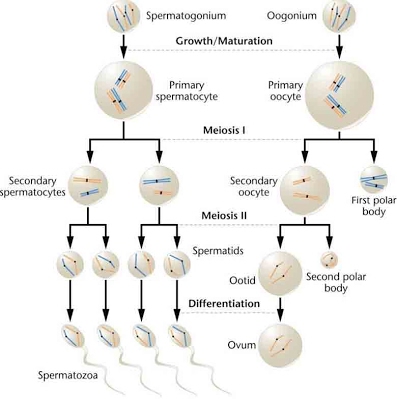
Meiosis II sees the secondary oocytes and spermatocytes divide again and produce spermatids and ootids. For sperm, this gives 4 cells, but with another second polar body (actually 3 this division, not shown; so 4 overall counting the first polar body) produced alongside the first discarded polar body, there is only one egg per meiosis process.
Finally, mature gametes spermatozoa (spermatozoon, sg.) and ova (ovum, sg.) arise following the differentiation of the spermatids and ootids. Spermatozoa have developed flagella while ova have a thicker membrane – at this point fertilisation has occurred.
The process of making sperm, spermatogenesis, kickstarts at puberty. By this time, its counterpart oogenesis is already at the primary oocyte level. Ovulation is the step towards making the secondary oocytes.
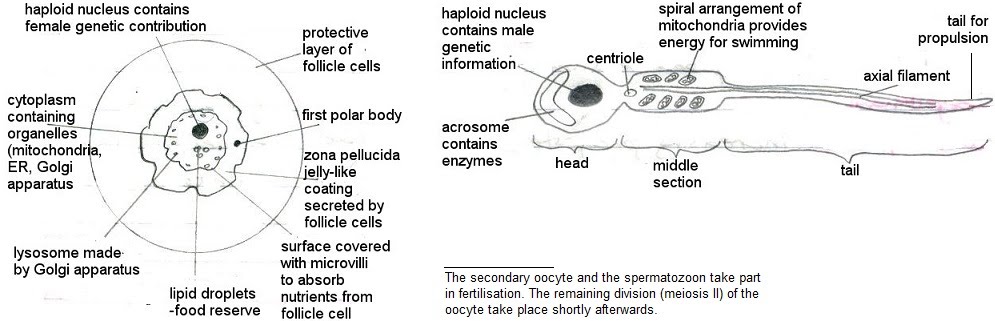
In the absence of fertilisation, oocytes stop here. Upon fertilisation, meiosis carries on to develop the ootid and ovum. This is now a zygote.
Hormone regulation of gamete development
Gamete development is a complex process orchestrated by multiple hormones, some of which are common between the male and female gamete development pathways.
Gonadotropin releasing hormone (GnRH) is secreted by the hypothalamus in the brain, which stimulates two further reproductive hormones to be produced in the anterior pituitary nearby: follicle stimulating hormone (FSH) and luteinising hormone (LH).
As the name suggests, FSH, together with LH, acts on the follicles in the ovary to stimulate their development as part of oogenesis. In the testes, FSH promotes sperm cell development in the seminiferous tubules while LH stimulates testosterone production by the Leydig cells.
The action of FSH and LH in the ovary results in the production of estrogen which prepares the uterus for pregnancy by thickening its wall and thinning the cervical mucus, as well as acting as an inhibitor for the further production of GnRH, FSH and LH.
In both pathways, inhibin inhibits production of FSH.
In the testes, testosterone maintains sperm production (testosterone is selectively accumulated here at levels tens of times higher than blood). As LH has already stimulated testosterone production, it simultaneously signals the decrease in secretion of GnRH, since its effect via FSH and LH has been accomplished. High testosterone levels are able to maintain sperm production without a need for further FSH and LH stimulation. Done.
Back to the ovary. The increasing estrogen at the point where the follicle has matured signals a large surge of LH and FSH (after initially being suppressed by estrogen… yes, confusing!) which results in ovulation – the egg is released. Done.
What happens next with hormones in the female pathway depends on whether fertilisation occurs.
Fertilisation
We’ve already treaded onto fertilisation territory, by describing the final developmental stage of eggs from secondary oocytes into ootids and ova. However, this is once fertilisation has occurred. Let’s step back and rewind in more detail the steps from the first contact of the two gametes to the moment their membranes fuse together to make one complete diploid cell – the zygote.
The sperm head contains enzymes that can break down the outer layer of the egg. It’s called hyalunoridase and the release of the sperm nuclear content initiates the further developmental responses by the egg including the release of the second polar body and preparing for division (cleavage).
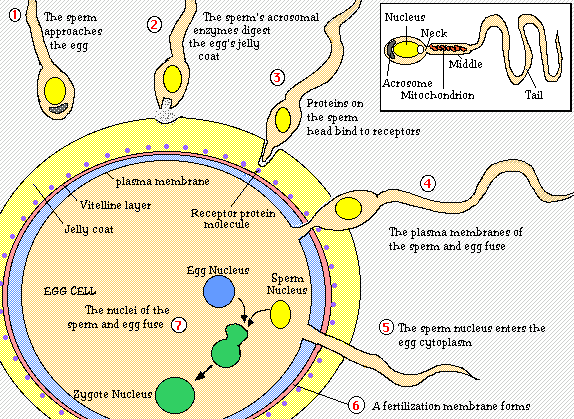
The tip of the sperm head is called acrosome. This is where the enzyme starts to break down the egg layer once it has approached the surface. Once the first sperm has broken down the egg coat and entered, the process is closed off to the remaining sperm cells.
Menstruation
We’ve seen that negative and positive feedback loops are fairly straightforward concepts. That’s because we were thinking of just one variable which either gets kept constant or gets increased/decreased continually. What happens when you have a complex interplay between 4 different variables?
The Mammalian Oestrus is Controlled by 4 Hormones: FSH, LH (pituitary gland), Progesterone and Oestrogen (ovaries)
FSH is follicle-stimulating hormone (it stimulates follicles to develop, which are immature eggs surrounded by other cells). LH is luteinising hormone (it stimulates the corpus luteum to develop which literally means yellow body and literally is a yellow body which secretes progesterone and forms from the “ashes”/remains of the follicle following ovulation).
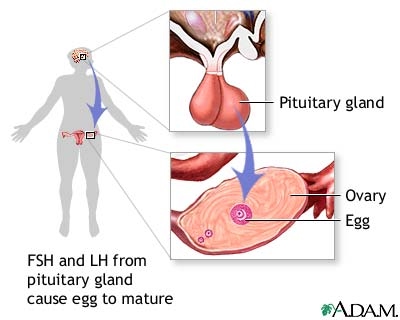
Here’s an overview of the entire cycle (it’s arbitrarily decided that day 1 is the first day of menstruation just because it’s easily spotted… no pun intended!):
1. Ovulation: a) high levels of FSH stimulate the ovaries to produce oestrogen
b) the follicle in the ovary gets stimulated by FSH to develop
c) increasing oestrogen levels inhibit FSH, but very high levels of oestrogen stop inhibiting FSH
d) oestrogen also stimulates LH secretion by the pituitary gland
e) FSH and LH peak, an egg is released
2. Luteal Phase: a) the corpus luteum formed from the remains of the follicle following ovulation secretes progesterone
b) increasing progesterone levels inhibits LH secretion
c) oestrogen stimulates, and progesterone maintains, the uterus lining in case of pregnancy
d) progesterone levels drop, and the cycle starts again
Bottom Line
The actual gory details aren’t necessarily something you must learn entirely. The purpose of this topic altogether is to exemplify negative and positive feedback. So let’s just focus on that at least, shall we? There are 3 feedback loops to take note of:
1. Negative feedback from oestrogen to FSH – hey FSH, I’m oestrogen and I know that you stimulated me in the first place, but how about you lay low now that I’m around. Until I get a bit more abundant! My presence means your absence for the time being, so tell that pituitary gland to stfu.
2. Positive feedback from oestrogen to LH – hey LH, I’m oestrogen and I want more of you. See how abundant I am? You want to be here with me, don’t you? Come on, come closer.
3. Negative feedback from progesterone to LH – hey LH, I’m progesterone and you’re making too much noise partying like that with oestrogen, so here’s the deal. Go away. Thank you in advance.
Pregnancy testing with monoclonal antibodies
Immobilised enzymes are central to various diagnostic reagent strip applications such as blood glucose monitoring and pregnancy testing.
These are antibodies which can be cloned from a single cell to make a high amount of them. They can bind to pretty much any substance, and are used in pregnancy tests as well as cancer treatment.The process involves taking a cell which produces antibodies such as a lymphocyte, and crossing it with a tumour cell. Tumour cells divide uncontrollably, so the end hybrid cell will produce many antibodies via its many clones.
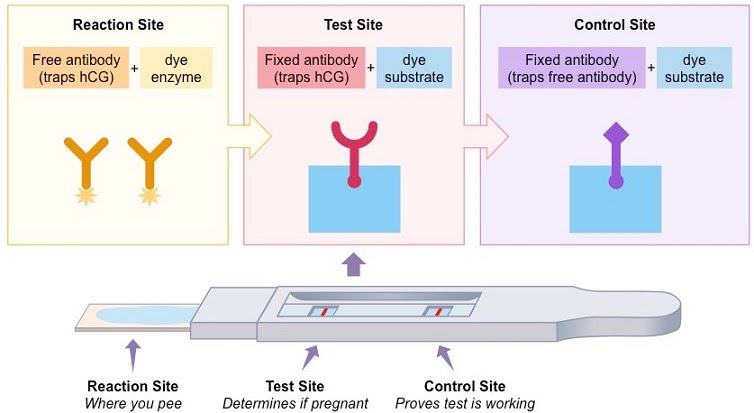
Human chorionic gonadotropin (hCG) is secreted early in embryonic development. Its presence in urine is indicative of pregnancy.
The high specificity of the antigen-antibody bond makes use of antibodies as a lab technique high on the list. Antibodies are used with proteins as a labelling method to detect presence of the target moiety (in biochemical reactions, testing or disease detection), or with whole tissue samples to detect presence of specific organelles for visualisation under a microscope.
Infertility
Issues at various levels of the reproductive system and process can hinder or prevent fertility, causing infertility.
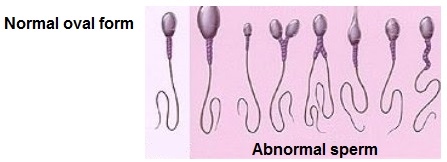
Low sperm count, shape and size, as well as obstructions of the vas deferens are causes of infertility. The threshold that is considered significant for infertility in terms of percentage of sperm with normal morphology is less than 60%. Therefore, up to 40% of sperm are expected to be deviant from normal morphology in terms of size, shape, tail and the midsection.
Sperm motility and vitality are also taken into account when assessing infertility. Motility refers to the ability of sperm to move, while vitality refers to the ability of sperm to stay alive long enough to potentially fertilise an egg as a result of ejaculation during sexual intercourse.
The vas deferens connects the testis to the seminal vesicle for the sperm cells to join the semen fluid prior to ejaculation. The sperm content will be low if this route is blocked. Causes of blockage include inflammation due to infection e.g. gonorrhoea, developmental issues that impact the shape or integrity of the vas deferens, and trauma e.g. from surgery on the testes. Intentional blockage is a procedure done as a form of birth control (vasectomy).
Ovulatory disorders, oviduct blockage, endometriosis and anti-sperm antibodies are counterpart infertility causes.
Ovulatory disorders mean that ovulation fails to take place, or occurs sporadically due to a perturbed menstrual cycle. This can be caused by a hormonal imbalance, such as hypothyroidism or hyperthyroidism, lifestyle factors or PCOS (Polycystic Ovarian Syndrome).
Oviduct (Fallopian tube) blockages, similarly to vas deferens blockages, prevent the gamete (egg) from travelling towards the fertilisation point further out from its origin in the ovary. Blockages are caused by different types of infections. As with the vas deferens, intentional blockage is a form of contraception.
Endometriosis is a condition where endometrial (womb lining) tissue grows elsewhere e.g. on ovaries, the bladder and other abdominal organs, or even further out. It can cause severe symptoms such as pain and extreme bleeding, or none at all. Endometriosis causes infertility by making it more difficult to become pregnant, or preventing it altogether.
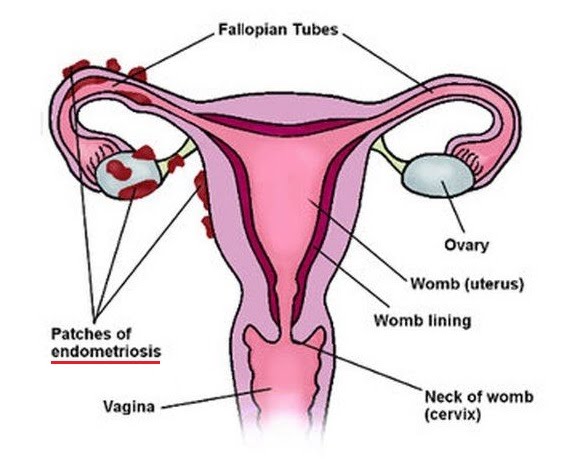
Anti-sperm antibodies develop when blood cells part of the immune response come into contact with sperm cells, identifying them as non-self. Usually, this does not happen because of the blood-testis barrier formed in the testes by the Sertoli cells, as well as the environment in the vagina which shields from the immune response. If these barriers are broken through cuts, surgery, etc. then antibodies may start being produced in the sperm host or recipient. This results in diminished sperm content of semen, or if the response takes place in the recipient, clumping of sperm cells or attack by T cells. This results in a decreased probability of sperm cells reaching the egg for fertilisation.
Assisted reproduction
To address some of these infertility issues, multiple approaches exist under the umbrella of assisted reproduction, or assisted reproduction technology.
The specific technology used depends on the cause of infertility. For example, low sperm count might prompt a surgical sperm retrieval to be used as part of IVF (in vitro fertilisation).
Ovulation disorders would prompt ovulation induction which would also be standard procedure for any egg-retrieving approach e.g. IVF or intra-cytoplasmic sperm injection.
Therefore, overlaps in technologies used and variations of each depend on the specific case of the person(s) attempting pregnancy. This is an overview of some of the technologies practised.
Ovulation induction is done through multiple treatments such as FSH (follicle stimulating hormone)-stimulating tablets e.g. clomiphene or tamoxifen, or directly via injection of FSH e.g. Menopur, Puregon.
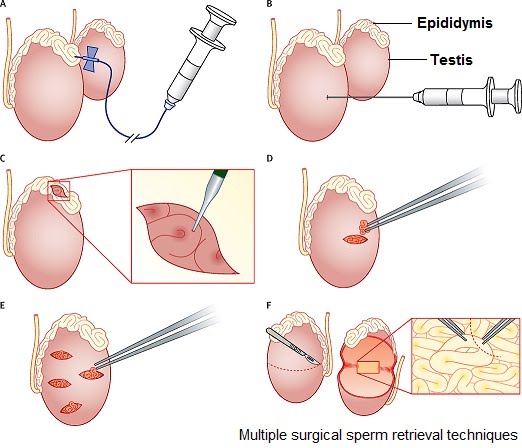
Surgical sperm retrieval is useful if sperm count or quality are low, or in the case of vasectomy or other blockages that prevent sperm being present in semen. It involves the aspiration (sucking out) of sperm from the tissues they develop and are stored in i.e. testis tissue or the epididymis.
Intrauterine insemination involves the delivery of sperm into the uterus via a thin tube. The sperm can be the prospective parent’s sperm, or donor sperm from a licenced clinic or an informal donor. Intrauterine insemination is helpful if sperm cannot make its way there due to low sperm counts or low motility, unexplained infertility or ejaculation dysfunction. Other reasons include inability to have vaginal sex, or having a sexually transmissible infection such as HIV.
In vitro fertilisation (IVF) involves carrying out fertilisation outside the body by removing both eggs and sperm. This entails undergoing a complex treatment plan to induce the production of multiple eggs by the ovary, then collecting them. Fresh or frozen sperm is used from the parent or donor to fertilise the eggs in the lab.
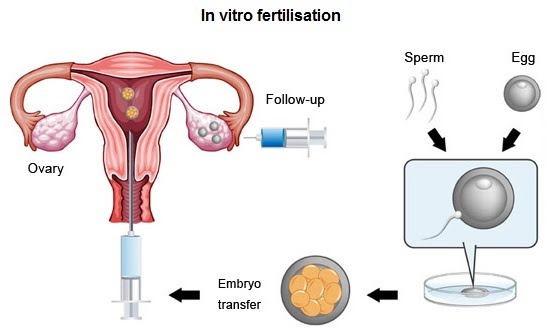
The top quality embryo is then implanted in the womb. Depending on how good the embryo is and the age of the carrier, one or two embryos are implanted. A good embryo has a better chance of developing alone. Multiple embryos (3 or more) are not implanted due to the increased risks to themselves and the carrier should they all implant and grow. A better approach is to freeze remaining good embryos for further implantation cycles. This is termed frozen embryo replacement.
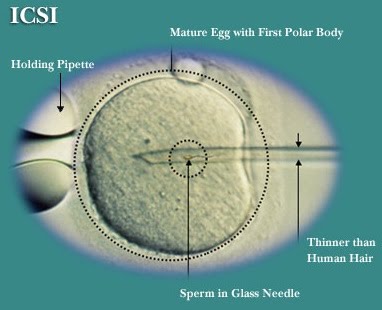
A technique that can be used alongside IVF is intra-cytoplasmic sperm injection (ICSI-IVF). Unlike IVF only, where multiple sperm are added to the egg to allow for fertilisation to happen spontaneously, ICSI involves the injection of a single sperm into the egg. This is useful if the sperm are unable to fertilise the egg. A potential loophole with ICSI is for heritable male infertility to be carried forward to male children (research ongoing).
Gamete intra-fallopian transfer (GIFT) is a “vanilla” version of IVF which places the egg and sperm in the Fallopian tube, allowing fertilisation to occur by itself. It may involve other steps such as ovulation induction, surgical sperm retrieval or donor sperm insemination. Instead of fertilising outside the body and implanting an embryo in the womb, GIFT only handles the gametes and leaves them in the Fallopian tube, where they would encounter each other and carry out fertilisation in a non-assisted reproduction scenario. Therefore, this is an appealing technique for those with specific beliefs around reproduction, which make IVF unappealing e.g. picking the embryo to be implanted.
Ok byeeeeeeeeee
